For Butane we have a total of 26 valence electrons. Do not consider cyclic ring structures.

Draw Two Lewis Structures For A Compound With The Formula C4h10 No Atom Bears A Charge And All Brainly Com
According to IUPAC nomenclature these isomers are called simply butane and 2-methylpropane.

. Draw the Structures of Possible Isomers of Butane C4h10. There are two isomers. Chains Branches and Rings of Carbon Compound.
We will use this approach in our subsequent examples. Finally the only choice for a branched-chain ether is. This is the C4H10 Lewis structure.
Draw the structures of possible isomers of butane C 4 H 10. Draw two lewis structures for a compound with the formula c4h10. Asked on 13th Jan 2021 a Write the IUPAC name and common name of CH3Clb Draw the structure of chlorobutanec Draw the structure for bromopentane.
No atom bears a charge and all carbon atoms have complete octets. Butane and 2-methylpropane isobutane. Explain why the hydrocarbon you chose in part b has the higher boiling point.
Advertisement Remove all ads. Notice that isobutane has a propane parent chain with a methyl group - CH3 attached to the second carbon of the chain - that is why its IUPAC name is 2-methylpropane. Advertisement Remove all ads.
Isomers Of C4h10 Structural Formula - 18 images - ppt 4 types of isomers powerpoint presentation free the number of structural isomers possible fromthe lecture problem draw all the constitutional isomers of draw the isomers of. Circle one C4H10 C6H14 c. Use your diagram to answer the following questions.
The total valence electron is available for drawing the Butane C4H10 Lewis structure is 26. Draw Lewis structures for the linear hydrocarbons C4H10 and C6H14. Draw two lewis structures for a compound with the formula c4h10.
C4H10 We finally encounter an example where there is more than one possible carbon skeleton. That makes it a little bit easier to draw the C4H10 Lewis structure. The lewis structure of C4H10 has 13 bonding pairs and zero lone pairs.
The molecular geometry of C4H10 with respect to the carbon atom is tetrahedral. Label your claim evidence and reasoning. No atom bears a charge and all carbon atoms have complete octets.
- 4985972 ryanwilliamson8972 ryanwilliamson8972 09022017. Which do you predict to have the higher boiling point. A Why does the element carbon form a large number of carbon compoundsb Write down the structures and names of two isomers of butane C4H10.
There are 2 possible isomers of C4H10. - 5007612 neymar1909 neymar1909 09042017. There is more than one acceptable diagram with the same answers.
Well put four Carbons in a row and then well put Hydrogens around them. Answer 1 of 2. 1 on a question Draw as many unique lewis structures as possible for c4h10.
The number of C-H bonds The number of C-C single bonds The number of CC double bonds The total number of lone pairs Submit Answer Retry Entire Group 9 more. Propose all possible structures for a compound with molecular formula C 4 Propose all possible structures for a compound with molecular formula C 4 H 8 O that exhibits a broad signal between 3200 and 3600 cm -1 in its IR spectrum and does not. Whenever we see the ending ane we know that were going to have Carbons and Hydrogens single bonded.
Lets start with five atoms in a row. Draw a Lewis structure for C4H10. Drawing your structures with a straight chain as much as possible will give you the best chance of not overlooking a structure.
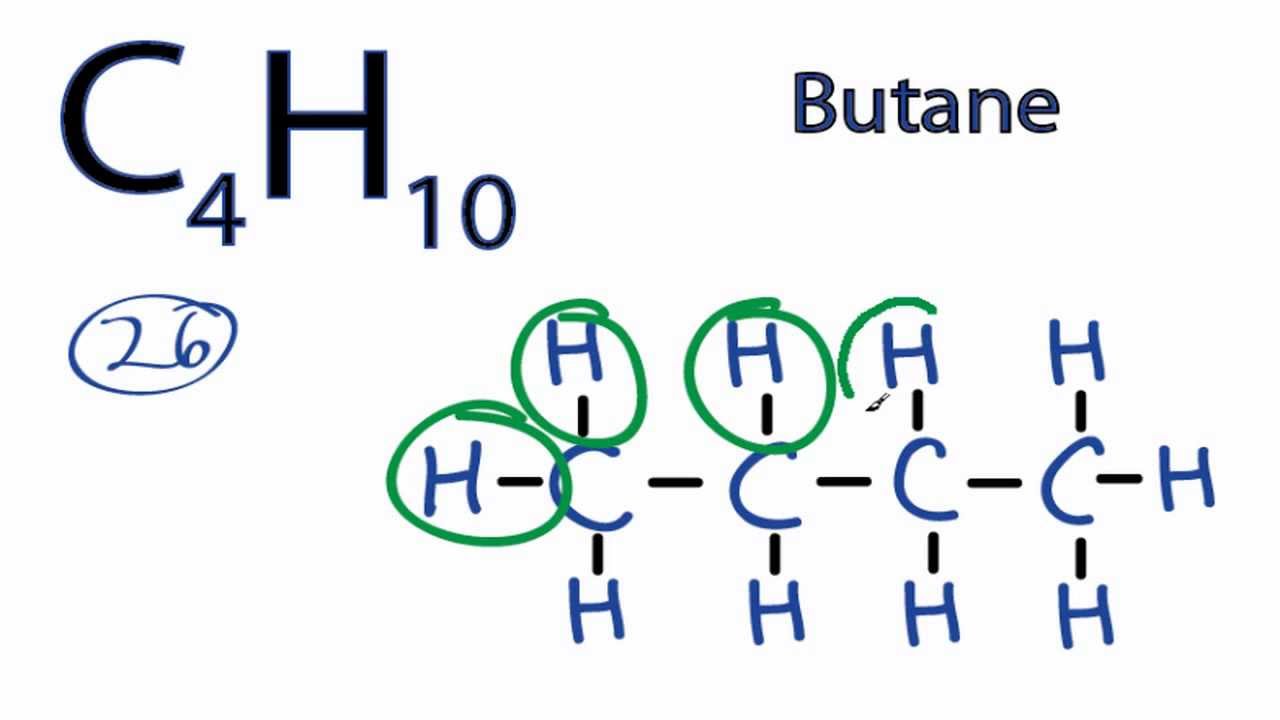
C4h10 Lewis Structure How To Draw The Lewis Structure For C4h10 Youtube

What Are The Possible Isomerisms Of C4h10 Quora

Draw Two Lewis Structures For A Compound With The Formula C4h10 No Atom Bears A Charge And All Brainly Com
0 Comments